Organ Transplant Preservation
Background
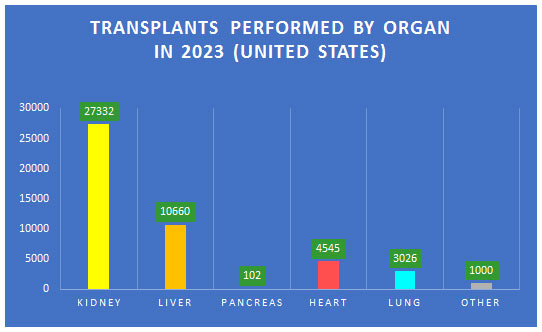
According to the Organ Procurement and Transplantation Network (OPTN), more than 46,000 transplants were performed in the United States in 2023. However, 17 people die each day waiting for an organ transplant and nearly 113,000 people are waiting for transplants. Transplanted organs have a very short shelf life, which can range from four to 36 hours. Most organs are placed in “static cold storage” after donor removal. Prior to placing the organ in cold storage, the organs are flushed with a preservation solution to protect the organ from damage caused by extreme cold.
At normal body temperatures, the body’s own cells pump various ions and other substances into and out of their cytoplasm to maintain physiological concentrations. Preservation solutions also contain nutrients and antioxidants to sustain cells and subdue inflammation. Cooling an organ helps delay the onset of ischemia, a condition in which organ tissues become damaged or dysfunctional due to the lack of oxygen.
Transplanted hearts lose viability faster than any other transplanted organs. At the four-hour mark, heart cell function begins to fail, and the likelihood of organ malfunction or failure rises significantly. Kidneys are the most resilient, which remain viable for 24 to 36 hours, but lungs remain viable for 6 to 8 hours and the liver has a shelf life of approximately 12 hours.
Organ transplants are time sensitive – a human heart and lungs have a four-hour shelf life to get from the donor to the recipient. |
Furthermore, flight delays present an additional burden on organ transportation from the donor to the recipient. Prior to 9/11, couriers could deliver organs directly into planes, often right to the cockpit, then another courier would meet the aircraft at its arrival gate to collect the package and deliver it to the hospital.
present an additional burden on organ transportation from the donor to the recipient. Prior to 9/11, couriers could deliver organs directly into planes, often right to the cockpit, then another courier would meet the aircraft at its arrival gate to collect the package and deliver it to the hospital.
Although 3,500-4,000 heart transplants are performed in the United States yearly, more than 100,000 patents could benefit from a new heart.
More than 10,000 hearts are donated each year in the United States, but approximately 3,000-3,500 are unusable due to a problem with the heart, or another comorbidity that precludes transplantation; and another 3,000-3,500 cannot be used expressly due to the inability to get the heart to the donor in time.
The Theratome Solution
A Theratome solution has been developed from stem cell secretions including exosomes, growth factors and cytokines, which repair and support preservation of organ and tissue viability. Data generated in collaboration with Dr. Pinar Zorlutuna’s laboratory in Notre Dame, supported by significant VA funding, has produced very promising results.
In the presence of Thera-103, human heart muscle cells show a 70 percent recovery in beat rate after exposure to cold ischemic “transplant transport conditions” for eight hours, as compared to a normal 50 to 60% recovery rate observed after four hours of cold ischemic incubation with the current clinical standard organ preservation solution alone. These results suggest that the addition of Thera-103 could effectively double the length of the transport time window. Logistically, a small vial of this product can be added to the standard transport solution and to the heart, resulting in the arrival of a healthier heart.
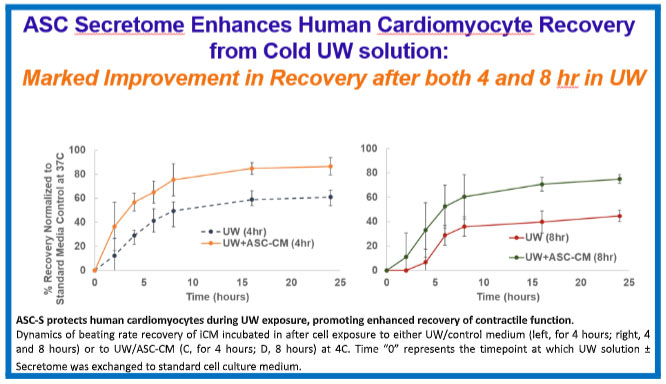
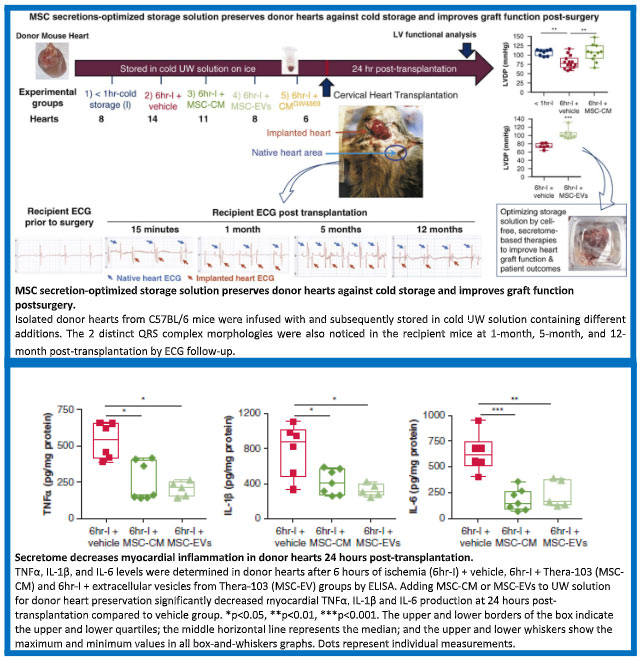
Because healthy human hearts are not available for experimentation, rodent hearts were exposed to cold ischemic transport solution with or without Thera-103 for an elongated time of 6 hours, and then implanted into recipient mice in a heterotopic transplant procedure, as summarized below.
The hearts that had been treated with Thera-103 showed more rapid recovery of function and markedly fewer dead cells, just as observed with rodent hearts ex vivo, and also with the human heart cells as shown above. In concert with these findings, use of the modified transport solution containing Thera-103 also significantly reduced the inflammatory factors found in the heart post-transplantation, as shown below.
Competition
Currently, Thera-103 has no direct competitors. The shelf-life limitations of current transport solutions are well known. This product is additive to the current treatment paradigm.
