About Us
Leadership
Investors
Commercial Opportunities
- Medical Need – The Theratome platform technology captures and concentrates therapeutic factors produced by adipose stem cells (i.e., the Secretome) into a defined formulation of concentrated therapeutic factors that provide precise dosing and immediate bioavailability compared to cell therapy. Because the cells’ Secretome is recognized as being responsible for the regenerative and protective effects derived from these stem cells, our technology represents a significant advancement in cell-based therapeutic science and patient treatment.
- Safety – Secretomes are produced by cells derived from qualified human donor fat (adipose) tissue cell lines with a final biologic drug composition that is void of potential toxic excipients. Experiments in a broad range of animal models have not revealed any safety concerns when Secretome is administered via intravenous, local, or subcutaneous route.
- Indications – Secretomes comprise a platform of products that can be used to treat many disease conditions, especially diseases where organ ischemia and/or inflammation are implicated.
- Synergy – Secretomes can be combined with other treatment modalities to achieve optimal outcomes.
- Manufacturing – Secretomes are produced using a scalable process that can readily be tech-transferred to a contract manufacturer. They consist of allogeneic off-the shelf products generated from working cell bank cultures with final formulations that are stored and transported under non-cryogenic frozen conditions or as a room temperature-stable lyophilized powder.
- Proprietary Position – Secretomes are protected by 6 USPTO-issued patents with two additional patents pending in the United States. In addition to adipose-derived Secretomes, the patent portfolio also covers other mesenchymal stromal / stem cells such as those derived from bone marrow and umbilical cord tissue. These therapeutics have eligibility for Orphan Drug exclusivity in the US (granted by FDA), Europe, and Japan.
- Regulatory Approach – Depending on the disease indication and claimed use, Secretomes may be approved as either a biologic drug, a medical device, or combination drug/device product. They may also receive Fast Track, Humanitarian Use Device (HUD) and eventually Regenerative Medicine Advanced Therapy (RMAT) designations.
- Artificial Intelligence (AI) – AI has the potential to optimize patient efficacy by predetermining which patients should benefit from a secretome treatment. For example, millions of patients are commonly misdiagnosed with dry eye disease, resulting in poor response to treatment.
The Secretome product line offers important clinical advantages including tailored formulations for specific diseases, rapid bioavailability of the beneficial therapeutic factors, consistency of manufactured product, and simplified storage compared to viable cell or tissue therapeutics.
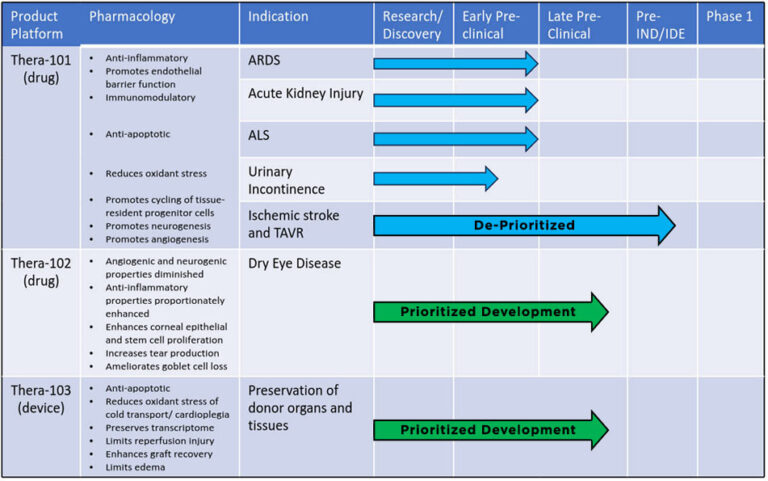
The Company is currently focused on advancing its pipeline in organ transplant preservation and dry eye disease. Should Theratome have additional financial resources, the active pipeline could include ARDS, ALS and Stroke.
Click on Individual Boxes to Learn More
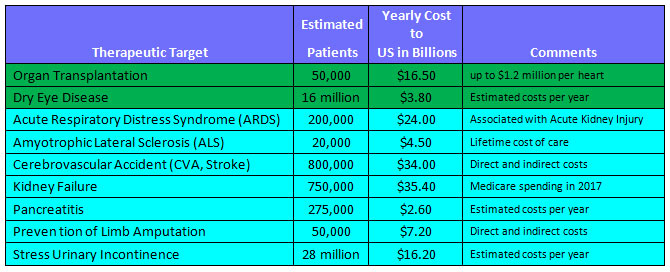
Target Market Sizes
Summary Table of Diseases Addressable by Theratome Technology, and Their Economic Impacts
Pipeline