About Us
Leadership
Investors
Our Science
 Theratome has developed a platform technology comprised of various biological factors expressed by stem cells and secreted into the body. These secretome factors consist of proteins, micro-RNAs, extracellular vesicles/exosomes and metabolites. Theratome’s founder, Dr. Keith March, discovered the potential therapeutic activity of adult stem cell-derived secretomes, which were initially described as “paracrine factors”, published in 2003 and 2004 (See Publications). However, the nomenclature used in this emerging field is still evolving. While secretomes were initially described as paracrine factors, they have also been referred as conditioned media (CM), therapeutic factor concentrate (TFC), and concentrated secretome (CS).
Theratome has developed a platform technology comprised of various biological factors expressed by stem cells and secreted into the body. These secretome factors consist of proteins, micro-RNAs, extracellular vesicles/exosomes and metabolites. Theratome’s founder, Dr. Keith March, discovered the potential therapeutic activity of adult stem cell-derived secretomes, which were initially described as “paracrine factors”, published in 2003 and 2004 (See Publications). However, the nomenclature used in this emerging field is still evolving. While secretomes were initially described as paracrine factors, they have also been referred as conditioned media (CM), therapeutic factor concentrate (TFC), and concentrated secretome (CS).
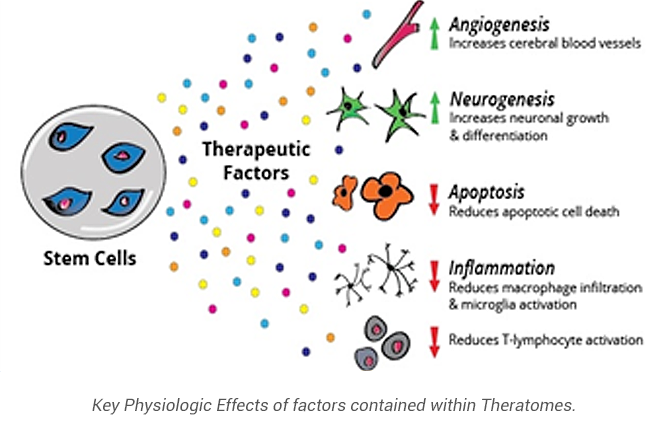
The principal activities of these factors include promoting angiogenesis and neurogenesis, modulating immunity, promoting progenitor cell formation, and limiting apoptosis. These activities synergize to “rescue and repair” of tissues from ischemic and inflammatory challenges that are key components of many disease pathologies.

Theratome Bio’s secretome products are manufactured from master cell banks under quality control conditions yielding clinical grade products. When derived from adipose stem/stromal cells, these factors have been referred to as ASC-CM or ADSC-CM, ASC-S, ASC-CS, etc… Similar naming conventions have been used when secretomes are derived from mesenchymal stem/stromal cells from bone marrow (MSC), whole blood, or from umbilical cord blood (e.g., USC, or ULSC).
From the secretome platform, products are further refined, optimized and formulated for specific disease conditions. For example, for dry eye (Thera-102), Theratome Bio’s product is an eye drop, evaluated as a biologic drug under the auspices of the FDA (CBER). Furthermore, the angiogenic and neurogenic properties are selectively minimized/deleted, and anti-inflammatory components are optimized. Theratome filed a patent for this new product in August 2023.
Secretomes are a precision medicine formulated to target specific disease processes. Therefore, patient selection can aid in targeting disease which is directly addressed by using AI to aid in identifying the disease process and the appropriate patients. Initially AI models are being developed to identify patients with Dry Eye since current clinical tools lead to a 40% rate of mis-diagnosis.
For organ preservation, the secretome product (Thera-103) is evaluated as a biologic device under the auspices of the FDA (CBER). It should become a supplement/additive to current transplantation solutions. In practice, the preservation solution containing Thera-103 is flushed out prior to transplantation into the patient. Tissue viability markers can be assessed with Multi-omics and the libraries assessed to determine the viability of tissues. Il-RNA and IL markers have been closely linked with viability.
Theratome has six issued US patents and another two pending US patents.
