Cerebrovascular Accident (CVA) / Stroke
Background
Stroke is the second most common cause of death in the United States and is the leading cause of long-term morbidity for adults. Approximately 20 million people worldwide suffer from stroke each year. In the United States alone, the number of cases is about 800,000 per year. Strokes can be classified as either ischemic (interruption of the blood supply to the brain) or hemorrhagic (rupture of a blood vessel or an abnormal vascular structure); approximately 87% of strokes are ischemic. When the brain is deprived of oxygen (ischemia) due to a blood clot or atherosclerotic plaque lodging in the brain, the result may be a stroke or impairment in cognitive function that can occur even when the ischemic lesion is not clinically evident.
The greatest risk factors are high blood pressure, high blood cholesterol, obesity, smoking and heavy alcohol use. Other factors include use of amphetamines and cocaine. Diabetes increases the risk of stroke by two to three-fold.
Some of the physical disabilities caused by stroke include muscle weakness, pressure sores, incontinence, appetite loss, inability to conduct activities, speech loss, vision loss and pain. Severe strokes can result in coma or death.
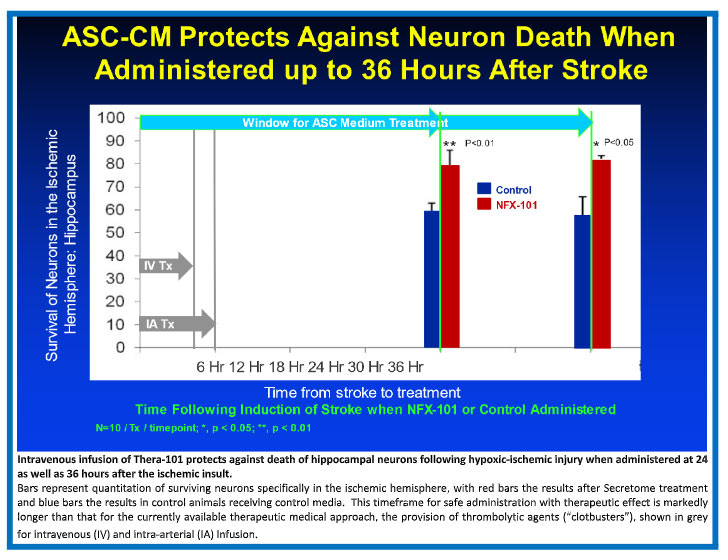
The use of the approved medication for acute stroke, tPA, is generally limited to only 5% of stroke victims due to poor effectiveness as well as the increased risk of intracranial bleeding as a deadly complication, if tPA is administered more than 4 hours after the stroke.
The transcatheter aortic valve replacement (TAVR) procedure was performed on an estimated 73,000 patients with aortic stenosis in the US and Europe in 2019 and is experiencing rapid growth. TAVR has been shown to prolong life in these patients and is an increasingly common procedure. However, asymptomatic ischemic brain lesions will occur in virtually all TAVR patients and strokes will occur in 1% – 2% of patients. These clinically silent lesions are believed to cause cognitive deficits in patients and form the rationale for development of products that will prevent these adverse events from occurring.
The Theratome Solution
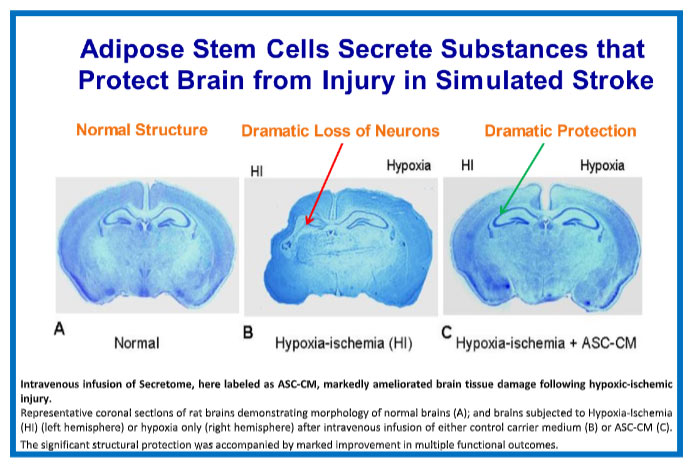
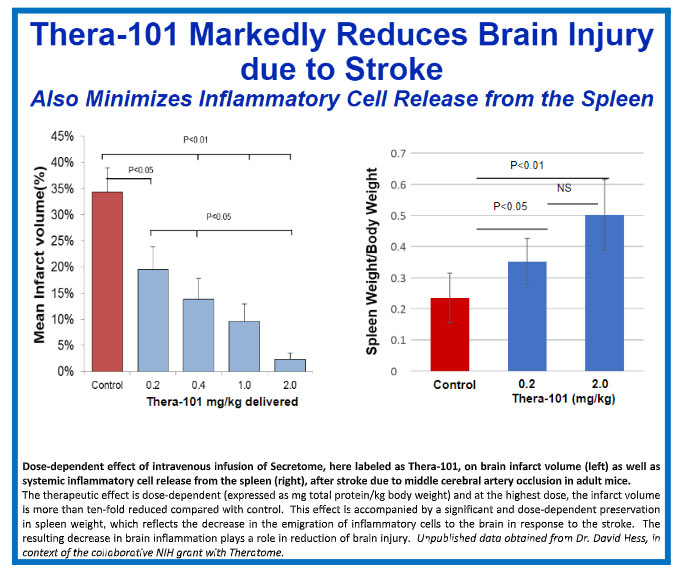
Our lead Theratome product, Thera-101, has been shown to protect and repair damage in preclinical animal models when delivered up to 36 hours after blocking blood flow to the brain (similar to the damage that results secondary to TAVR or ischemic stroke). This timeframe for safe treatment without promotion of hemorrhage makes extending this treatment to all stroke victims a real possibility. The Company intends to demonstrate a reduction in ischemic brain injury following TAVR in a proof-of-concept clinical trial, as an entry point for development of Secretome product for ischemic stroke and other ischemic brain injuries.
Clinical Trial Strategy
The Company’s operating plans reflect the FDA’s guidance in our pre-IND meeting covering manufacturing, preclinical studies and the Phase I proof-of-concept trial clinical trial in TAVR patients. The FDA meeting confirmed that following completion of standard 13-week preclinical safety studies in a single animal model (rats) to be conducted under GLP guidelines, and production and testing of a sufficient amount of Secretome to support finalization of the Chemistry, Manufacturing and Controls (CMC) as well as dosing for the clinical trial, the Company will be in position to file an IND for clearance to conduct a Phase 1 clinical trial. In brief, each cohort will have eight participants, randomized 3:1 between the dose for that cohort, and placebo saline infusion, following standard in-hospital TAVR. Thus, the final groups will provide for 6 participants in each of a control, low-, medium-, and high-dose group. The outcome variables will be clinical neurological testing and quantity and volume of new brain lesions as detected by diffusion-weighted MRI obtained one week after TAVR, in comparison with baseline neurological testing and MRI.
Competition
We believe there is currently no other company developing a secretome/paracrine factor concentrate product for the ischemic brain injury market. Embolic (or cerebral) protection devices (EPDs) are being developed by medical device companies to address this need. These devices are designed to capture plaque that has been dislodged by the TAVR devices and prevent ischemic lesions. However, a recent publication by Keystone Heart (for example) showed that its EPD device resulted in about 85% of patients with cerebral ischemic lesions and was not significantly different than the control groups. Early feedback from TAVR experts supports our belief that a biologic approach will be preferred to EPDs. Other companies are developing stem cell products for treatment of stroke; they all are in early clinical development stages.
