Dry eye disease (DED)
 According to the Tear Film & Ocular Surface Society Dry Eye Workshop, the definition of Dry Eye is:
According to the Tear Film & Ocular Surface Society Dry Eye Workshop, the definition of Dry Eye is:
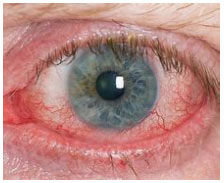
Dry Eye Disease is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.
Dry Eye Disease (Keratoconjunctivitis sicca or KCS) is a common condition that occurs when one’s tears aren’t able to provide adequate lubrication for your eyes. Tears can be inadequate and unstable for many reasons. For example, dry eyes may occur if you don’t produce enough tears or if you produce poor-quality tears. This tear instability leads to inflammation and damage of the eye’s surface.
While tear production decreases with age, hormones also play a role as more women suffer from DED than men, especially after menopause. Many cases of Dry Eye do not have a known underlying cause and are referred to primary disease.
Secondary causes of dry eye in humans include:
- Autoimmune Diseases, such as:
- Rheumatoid arthritis – Dry eye can quickly progress that could result in significant vision loss.
- Sjӧgren’s syndrome
- Thyroid disease
- Lupus – can lead to gritty feeling and burning of the eyes, which can lead to vision disturbance or loss, often accompanied with Sjӧgren’s syndrome.
- Scleroderma – causes a hardening of connective tissue, including the eyelids, resulting in decreasing tear production.
- Diabetes – Nearly half of diabetes patients suffer from dry eye due to a loss of nerves in the cornea, leading to a reduction of tear-producing lacrimal glands.
- Rosacea – half of patients develop ocular rosacea, with accompanying meibomian gland dysfunction that leads to rapid evaporation of tears.
- Graft versus Host Disease (GVHD), which is a major complication relating to allogeneic hematopoietic stem cell transplantation. While all parts of the eye may be affected, ocular GVHD is common. It causes destruction and fibrosis of lacrimal glands and conjunctiva, often leading to severe dry eye disease and decreased quality of life. While any conditioning agent for stem cell transplantation can cause this complication, it is most often associated with patients receiving total body irradiation. In the United States, more than 20,000 patients receive allogeneic stem cell transplantation each year and another 30,000 in Europe.
According to Eye Health Statistics, a total of at least 16 million Americans suffers from DED; other estimates are significantly higher. The prevalence tends to increase with age, with higher rates observed in postmenopausal women.
Dry Eye Disease imposes a substantial economic burden on the healthcare system and society due to both direct and indirect costs. The direct costs of DED include both medical and surgical expenditures. The medical costs associated with DED include diagnostic tests, ophthalmic appointments, prescription medications (e.g., artificial tears, anti-inflammatory drops), and other treatments. In severe cases of DED, surgical interventions such as punctal plugs or ocular surface reconstruction may be necessary. Due to the critical nature of sight to most aspects of daily life, its indirect costs are very significant, with both productivity loss and reduced quality of life. A study published in JAMA Ophthalmology estimated that the total annual cost of DED management in the United States exceeds $3.8 billion.
The Theratome Solution
 Currently, there is no definitive test in the clinic to definitely diagnose dry eye disease.
Currently, there is no definitive test in the clinic to definitely diagnose dry eye disease.
Multiple publications have demonstrated the ability of adipose-derived secretome/paracrine factors to preserve tear volume and protect corneal health in various pre-clinical models of dry eye syndrome. Previous studies have shown that adipose-derived secretomes decreased corneal epithelial defects, increased tear production, decreased goblet cell loss and reduced inflammatory cytokines production.
Experiments with our collaborators have extended these data by demonstrating that adipose-derived secretome/paracrine factors are also well-tolerated in an ex vivo rabbit corneal model (unpublished data).
Based on our experience with the broadly angiogenic properties of secretomes/paracrine factors, we have determined that an optimal Secretome formulation for chronic dry eye disease must have minimal angiogenic and optimal anti-inflammatory activity.
Treating dry eye disease with secretomes is not a novel idea. However, chronic use of secretomes/paracrine factors with high angiogenic activity could promote undesirable blood vessel formulation, especially with chronic use. A provisional patent was filed in 2023 for a unique ophthalmic 2nd generation Secretome (Thera-102) formulation to treat dry eye disease while reducing these potential off-target effects.
Artificial Intelligence
The use of AI during secretome development is a significant advantage because it accelerates development by in silico prediction of formulation and facilitates appropriate patient selection. It is estimated that as many as 40% of Dry is misdiagnosed, resulting in patients that are inappropriately treated. AI tools to identify patients can be developed as a standalone digital tool in advance of a Theratome product to ensure that the appropriate patients with Dry Eye will be identified for studies as well as accelerating product launch by incorporating this tool to better identify patients who would benefit from treatment.
Competition
The major pharmaceutical prescribed for this market is Restasis®/cyclosporin solution. It has minimal efficacy and costs up to $500 per month. Specifically, the FDA approved Restasis to increase tear production in 2003, after a 1999 application failed when reviewers and a unanimous FDA advisory committee concluded it did not meet efficacy criteria. Even though Restasis did not improve symptom scores (compared to placebo) when tested directly in the pivotal trials, the FDA accepted indirect evidence from the validation study in which, at six months, 15% vs. 5% of patients had a response with Restasis vs. placebo in a pooled analysis.
Restasis reached peak sales in 2022 ($2.3 Billion). However, revenues are expected to decline due to generics, which were first approved in February of 2022. The active ingredient in all of these products is cyclosporine.
Many patients also use autologous serum (platelet rich plasma) to treat dry eye disease, which is normally compounded at a pharmacy. This approach is non-selective, costly (up to $500 per month) and is normally not covered by insurance.
Restasis is a registered trademark of AbbVie, Inc. (ABBV).
Dry Eye Disease in Dogs
 Dry eye disease is a common disorder in dogs but is rare in cats. While studies have shown dry eye disease affects as many as 10% of the dog population, certain breeds are more susceptible to this disease condition. These breeds include the American Cocker Spaniel, Boston Terrier, English Bulldog, English Springer Spaniel, Miniature Schnauzer, Lhasa Apso, Pekinese, Pug, Shih Tzu and Yorkshire Terrier.
Dry eye disease is a common disorder in dogs but is rare in cats. While studies have shown dry eye disease affects as many as 10% of the dog population, certain breeds are more susceptible to this disease condition. These breeds include the American Cocker Spaniel, Boston Terrier, English Bulldog, English Springer Spaniel, Miniature Schnauzer, Lhasa Apso, Pekinese, Pug, Shih Tzu and Yorkshire Terrier.
Dry eye disease is the most common eye problem in dogs. While cases can range from mild to severe, left untreated can lead to scarring and vision loss, including blindness. Moderate to severe cases will generally manifest with red eyes and thick mucoid discharge.
 Possible causes of dry include congenital issues, large bulbous eyes in certain species that prevent full closure of eyelids (Pekinese, pugs), nerve damage and autoimmune disease. Autoimmune disease is the most prevalent cause of dry eye in dogs. This autoimmune response causes the immune system to attack the lacrimal glands and shuts down its tear-producing function.
Possible causes of dry include congenital issues, large bulbous eyes in certain species that prevent full closure of eyelids (Pekinese, pugs), nerve damage and autoimmune disease. Autoimmune disease is the most prevalent cause of dry eye in dogs. This autoimmune response causes the immune system to attack the lacrimal glands and shuts down its tear-producing function.
The most common diagnostic for dogs is the Schirmer tear test, which consists of a blotting paper used to soak up tears. The amount of fluid absorbed over a minute-period of time would determine the severity of the dry eye disease.
The most common treatments for dogs include cyclosporine and pilocarpine eye drops.
KCS Trial in Companion Dogs
Theratome Bio has initiated a clinical trial to treat companion dogs with KCS. In this pilot trial, 10 dogs will be treated and evaluated over three months. This trial should be completed later in 2025.
Following the completion of this trial, the Company intends to conduct a pivotal trial in dogs and to initiate a human trial to treat patients with KCS.
